Luminescent Reagents Calcium Detection (Aequorin)
AB-2900 Aequorin
Bioluminescent detection of Calcium
Aequorin is a photoprotein and a useful tool in biochemical and molecular biology for the measurement of calcium levels (concentration).
Purpose and Application
Aequorin can be used for detection of intracellular calcium ion concentration changes.
Features
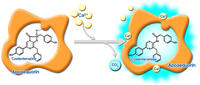
Aequorin is a photoprotein(1) isolated from luminous jellyfish(aequorea aequorea) and exists as a molecular enzyme complex composed of apoaequorin(apoprotein) and coelenterazine (luminescent substrate). This complex reacts with calcium ion and generates blue light that peaks at 470nm and therefore can be utilized for detection of the changes in intracellular calcium ion concentration under physiological conditions(4)(5)(6).
Since Aequorin does not pass through the cell membrane, it is introduced in the cell by the method that temporarily accelerate the membrane permeability(7) or by microinjection. Once the intracellular calcium ion concentration isn increased in response to same kind of stimulation,aequorin immediately reacts to this change and emits light.Unlike fluorescence detection, it produces specific luminescence that is detectable without excitation energy such as UV light, and there is no concern about autofluorescence.The introduced aequorin evenly distributed throughout the cytoplasm without migrating into sub cellular organelle, endoplasmic reticula or extracellular space, allowing for monitoring of changes in calcium ion concentration over long time couse.
The product provided as a purified recombinant aequorin with apoaequorin gene produced by cloning(2)(3), and therefore higher in purity and more stable than natural products.
Specifications
| Product Number | AB-2900 |
|---|---|
| Product Name | Aequorin |
| Material | Recombinant Aequorin |
| Purity | More than 95%(verified by CBB staining after SDS-PAGE) |
| Package | 50ug |
| Storage | -80°C |
A manufacturer: Chisso Corporation
Reference
(1) Shimomura, O., Johnson, F. H. and Saiga, Y., J. Cell. Comp. Physiol., 59 223-240 (1962)
(2) Inouye, S., Noguchi, M., Sakaki, Y., Takagi, Y., Miyata, T., Iwanaga, S., Miyata, T. and Tsuji, F. I., Proc. Natl. Acad. Sci. USA, 82 3154-3158 (1985)
(3) Shimomura, O. and Inouye, S., Protein Expr. Purif., 16 91-95 (1999)
(4) Blinks, J. R., Mattingly, P. H., Jewell, B., R., Leeuwen, M. V., Harrer, G. C. and Allen, D. G., Methods in Enzymology, 57 292-328 (1978)
(5) Blinks, J. R., Methods in Enzymology, 172 164-203 (1989)
(6) Miller, A. L., Karplus, E. and Jaffe, L. F., Methods in Cell Biol., 40 305-338 (1994)
(7) Snowdowne, K. W. and Borle, A. B., Am. J. Physiol., 247 C396-408 (1984)
Documents
| AB-2900 Aequorin Brochure |
|
| AB-2900 Aequorin Manual |
|



